The First Ever Treatment For COVID-19 Was Just Approved In Canada (VIDEO)
This is huge news! A COVID-19 treatment in Canada that was just approved is the first one ever to get the green light here. With the drug, people with severe symptoms related to the virus can get some relief.
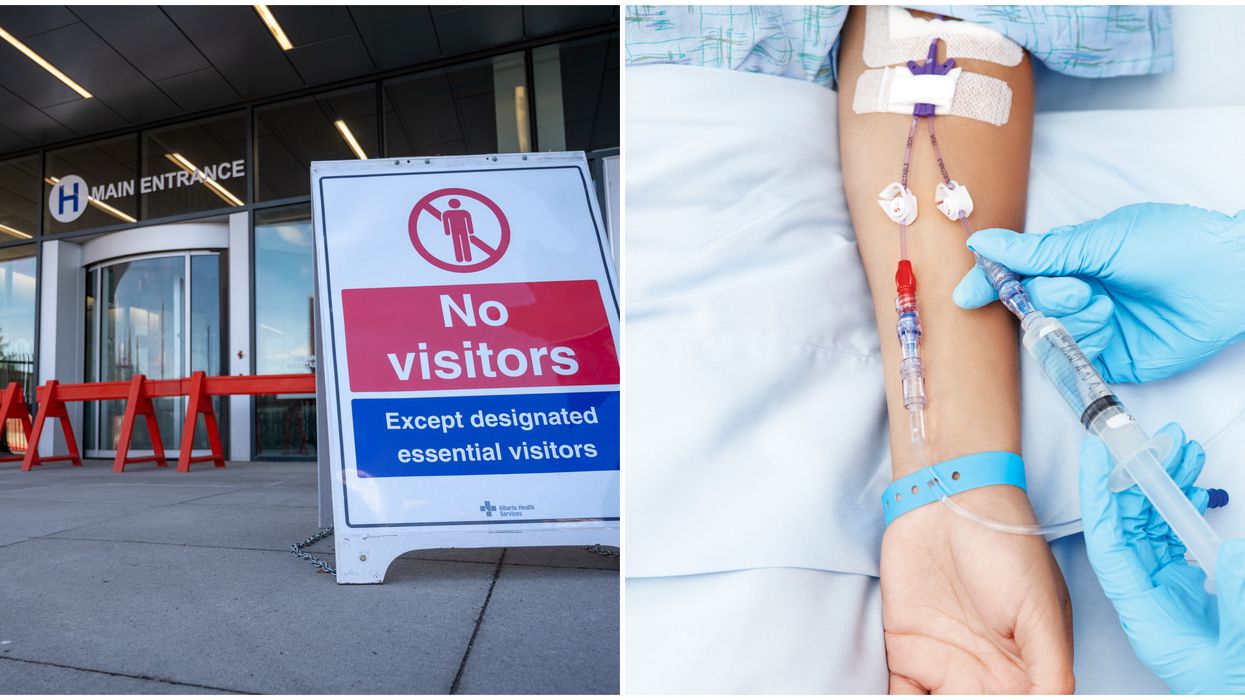
On July 27, Health Canada authorized a drug made by a Canadian manufacturer for use. It's called remdesivir, which goes by the brand name Veklury.
Editor's Choice: Canadians Can Now Go To Barbados & Work Remotely From There For The Next Year
It will treat patients with severe COVID-19 symptoms who have pneumonia and need extra oxygen to help them breathe.
Currently, the drug can only be administered to adults and adolescents who are more than 12 years old and weigh at least 40 kilograms. That's about 88 pounds.
It is given through an IV and will be used only in health care facilities where patients can be monitored closely.
This is the first drug that Health Canada has approved for the treatment of COVID-19.
The national health agency will be looking at the safety of remdesivir continuously and take action if there are any safety concerns.
This is a conditional approval — that means the manufacturer has to ensure the continued safety, effectiveness and quality of the drug.
The authorization done by Health Canada did include a full scientific review.
The manufacturer also has to provide Health Canada with safety monitoring reports along with reports on any and all serious reactions to the drug.
More data on its safety and effectiveness also needs to be sent over which includes final data from clinical trials.
The Public Health Agency of Canada is working with the manufacturer to make sure that access to the drug is secured for Canadians.
There was an expedited six-week review of the drug that was done which determined that its benefits outweigh its risks.
Remdesivir has also been given emergency or conditional authorization in the U.S., Japan, Singapore, Australia and Europe.
A Canadian company has started human trials for a plant-derived COVID-19 vaccine and other work is being done all over the country to eventually produce one.