Acetaminophen Tablets Recalled In Canada Because A Label Error May Cause An Overdose Or Death
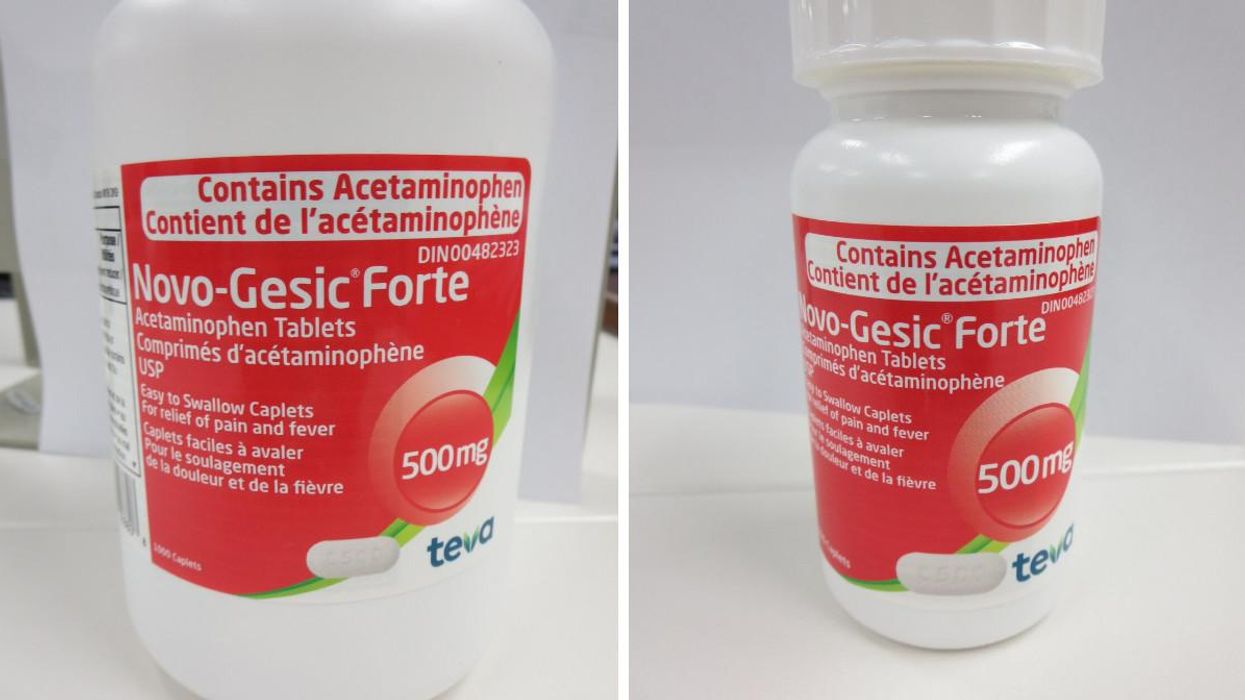
Bottles of Novo-Gesic Forte Acetaminophen Tablets are affected by the recall.
The newest recall in Canada to be aware of is regarding acetaminophen tablets that could cause an overdose or even death because of an error on the label.
On October 6, Health Canada reported that Teva Canada is recalling two lots of Novo-Gesic Forte Acetaminophen Tablets because the labelling error could lead to a person taking more than the maximum daily dosage for acetaminophen.
The bottle states a person shouldn't take more than nine to 12 tablets in 24 hours but the number of tablets should actually be eight.
The company has listed the affected products as Novo-Gesic Forte Acetaminophen Tablets that come in bottles and have a 500 mg strength.
With this recall, the 100-tablet bottle with the lot number 35364729A, DIN 00482323, UPC 068510028402 and expiry date of June 30, 2023, is affected. The product was first sold on August 3, 2021.
The 1,000-tablet bottle of the pills with the lot number 35217483A, DIN 00482323, UPC 068510028808 and expiry date of June 30, 2023, is also being recalled. Its first date of sale was September 8, 2021.
According to Health Canada, consumers who follow the incorrect directions on the bottles could ingest doses of acetaminophen ranging from 4,500 to 6,000 mg (or nine to 12 tablets) in 24 hours and experience symptoms of an acetaminophen overdose. Signs of this include nausea, vomiting, lethargy, sweating, loss of appetite and pain in the upper abdomen or stomach.
Abdominal pain could be the first sign of liver damage and might not be apparent for 24 to 48 hours. Liver damage can result in liver failure or even death in the most severe cases.
Health Canada is asking people to stop using the recalled products and return them to where they were purchased. If anyone thinks they or a family member have taken too much acetaminophen, they should call a local poison control centre right away.